
Science
Editor
1333 H Street, NW
Washington, DC 20005
Sir:
This is a letter to the Editor concerning the article written by Gary
Taubes (Science June, 1990). I request this be published in Science as
soon as possible in fairness to Texas A&M University in particular
and to the field of cold fusion in general.
The accusation made by Gary Taubes that cold fusion cells at Texas
A&M University were "spiked" with tritium can be easily tested. It
is not even necessary to trust the people at Texas A&M or Mr.
Taubes. All that is required is to add tritium to an electrolytic cell
and see if it behaves like the tritium claimed to be produced from cold
fusion. This has been done and the results show that the tritium
claimed to result from cold fusion can not be caused, at least in some
cases, by the addition of tritiated water. Unfortunately, many of the
cells at Texas A&M were not studied in sufficient detail to allow
this conclusion to be applied to every cell. Nevertheless, the reality
of tritium production as a phenomenon can not be challenged on the
basis of this accusation. I sent the results described herein to Gary
Taubes (4/9/90) before his article was published. Unfortunately, he
chose to ignore this information.
In order to
arrive at the above conclusion, two factors need to be compared. One
factor is based on the time history of tritium concentration in the
electrolyte and the other is based on the distribution ratio (tritium
in the evolved gas divided by that in the electrolyte).
Cells that are claimed to produce tritium show a characteristic pattern
of tritium production. After a time interval that can be as short as
several days or as long as several months, the tritium content of the
electrolyte begins a steady increase that lasts several days to
several weeks. Bursts in the production rate have been observed. After
the cell stops production, the tritium content rapidly decreases over
several days and approaches a constant loss rate. The initial loss of
tritium is caused by removal of dissolved DT gas from the electrolyte
by the constant production of D 2 gas at the cathode and the later loss rate is caused by dilution. Dilution results from
replacing the electrolyzed fluid and sampling specimen by heavy-water
having a lower tritium content.
Figure 1
shows this behavior for a cell (#4) studied at Texas A&M[1]. The
gas phase over this and several other cells at Texas A&M contained
a much higher tritium concentration for a brief time than did the
electrolyte. This indicates that the production rate of DT gas
exceeded the rate at which it could exchange with dissolved gas as the
evolved gas bubbled through the electrolyte. These bursts of tritium in
the gas correspond to measured increases in the tritium content of the
electrolyte. Therefore, some of the tritium generated at the cathode
was able to enter the electrolyte as DT and/or DTO. Because the
sampling interval was only daily and because of the unknown amount of
recombinate, it is not possible from this figure to compare the total
amount of tritium in the gas to that in the electrolyte. After the last
tritium burst, the tritium content of the electrolyte decreased and
approached a constant rate of decrease after several days.
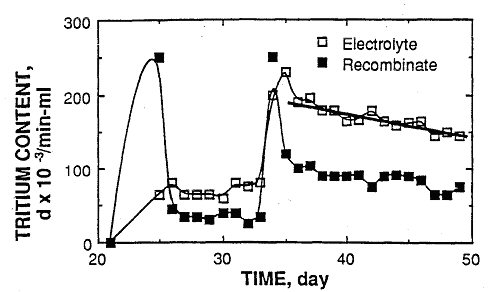
FIGURE 1. The plotted disintegration rate is that in
excess of the normal tritium content of the electrolyte. [1] No excess
tritium was seen until 23 days after electrolysis started. Excess heat
at a level near 18% was measured before, during and after tritium
production with an indication of bursts in heat production coinciding
with the tritium bursts. The light lines are drawn only to show how the
data are connected in time. |
Figure 2 shows the behavior of a cell studied at Los Alamos which was
in a group of active cells. Interestingly, cell #4 at Texas A&M and
the group at Los Alamos started producing tritium within 10 days and
stopped within one day of each other. In contrast to the Texas study,
the tritium production rate in the Los Alamos cell was so low that
excess tritium was not found in the gas phase. The same tendency exists
to show tritium bursts and to show a relatively rapid decrease in
tritium content over several days after production ceased.
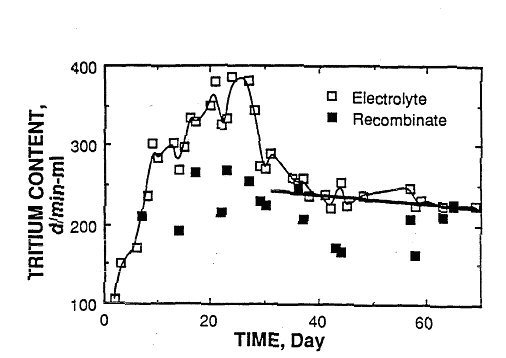
FIGURE 2. This was a sealed cell containing a catalyst which allowed
the gas to recombine and collect separately as the recombinate. Details
can be found in ref. [2]. All tritium entering and leaving the cell was
measured. The standard deviation of the disintegration rate for the
electrolyte is ±14 d/min-ml, about the size of the points. A larger
error is expected for the recombinate because of the way samples were
taken. The disintegration rate is plotted as a function of time after
electrolysis was started on 9/7/89. |
To test the behavior of a spiked cell, we added tritiated water to an inactive D 2
0 cell that had been running for 125 days without producing tritium.
After the tritium was added as HTO, a complete inventory was kept of
tritium that was added to the cell in the replacement D 2 0
and that left in the gas phase and during sampling. Figure 3 shows the
tritium concentration in the electrolyte as a function of time. [3] The
steady drop is caused by dilution when the electrolyzed D 2 0 was replaced by D 2 0 having a lower tritium content. In addition, a small amount of
enrichment would be associated with this process because the gas has a
lower T/D ratio than does the liquid. If these two factors are taken
into account, the excess tritium content of the electrolyte remains constant. This analysis shows that there is no mechanism to cause the
tritium content of the electrolyte to drop rapidly to a constant rate
of decrease if tritium is added as tritiated water. The addition of
tritiated water produces a constant rate of loss immediately after
its addition. Only the addition of gaseous tritium would produce
behavior similar to that observed in the cold fusion cells. It is hard
to believe that someone at Texas A&M would realize this behavior to
be characteristic of cold fusion cells, have HT or DT gas available and
have the equipment to bubble this gas through the electrolyte at just
the right rate.
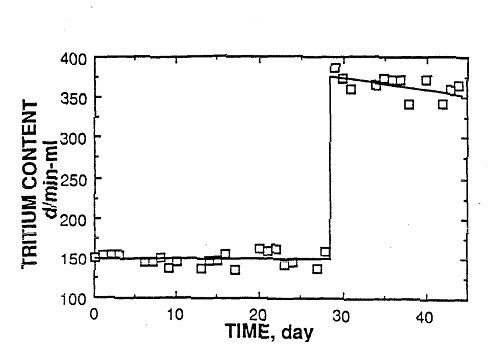
FIGURE 3. The standard deviation of the disintegration rate is ±14
d/min-ml. The disintegration rate is plotted as a function of time
starting at an arbitrary time. |
The appearance of a high tritium concentration in the gas phase (Fig.
1) also sheds light on this issue. Both experiment and theory agree
that the addition of tritiated water does not change the distribution
ratio significantly. Even extreme changes in cell conditions are found
to produce values between 0.45 and 0.65. Yet, several cells at Texas
A&M showed more than 100 times as much tritium in the gas as in the
electrolyte. Again, this behavior is not consistent with tritiated
water being added to the cells. Of course, tritiated water could have
been added to the collected recombinate to make the gas phase appear to
be high in tritium. The need to do this would have had to be recognized
and the amount chosen correctly at the right time. Although this
possibility can not be ruled out, it seems very unlikely to me. In
addition, if cell #4 had been spiked, the job would not have been
simple nor quickly done.
According to the cold
fusion group at the University of Utah, tritium has been produced by at
least 20 groups throughout the world. In addition, over 60 groups have
found some evidence for the cold fusion effect including heat,
neutrons, protons, gamma rays and X-rays. Some of this work, especially
in India[4] and Japan, has been very successful and is being published
regularly in Fusion Technology as well as in other journals. To suggest
that the cold fusion effect is not real because there is a suspicion
of fraud and contamination at one institution, is exceedingly
irresponsible.
| [1] |
J. O'M. Bockris, G. H. Lin and N.J.C. Packham, "A Review of the
Investigations of the Fleischmann-Pons Phenomena, Fusion
Technology, July (1990). |
| [2] |
E. Storms and C. Talcott, "Electrolytic Tritium Production", Fusion Technology, July (1990). |
| [3] |
E. K. Storms and C. Talcott, "A Study of Electrolytic Tritium
Production", The First Annual Conference on Cold Fusion, Salt Lake
City, Utah, 28 Mar. 1990. |
| [4] |
P. K. Iyengar and M. Srinivasan (ed.), "BARC Studies in Cold Fusion" Bhabha Atomic Research Centre, Trombay, Bombay, BARC-1500, Dec. 1989.
Will appear in Fusion Technology, July (1990). |

|

