|
|
|
| |
| New Energy Times Index of LENR Experimental Methodologies |
|
|
Introduction to New Energy Times Index of LENR Experimental Methodologies
Source: © New Energy Times. If you wish to reproduce any part of this index, please contact New Energy Times. Low-energy nuclear reaction researchers have used at least two dozen methods to perform LENR experiments. This index describes the more common methods. New Energy Times first presented a condensed version of this Index of LENR Experimental Methodologies at the American Nuclear Society meeting in November 2012. On May 22, 2013, we published a more detailed listing of the index here.
In the 1920s, when researchers searched primarily for transmutations, the typical experimental method they used was a high electric current rapidly introduced to a thin wire, causing it to explode.
When Martin Fleischmann and Stanley Pons reinvigorated the field in 1989 and searched primarily for excess heat, they used low-voltage electrolysis. Several months later, Francesco Piantelli developed an alternative method of loading hydrogen gas onto the surface of metals. Other researchers, as shown below, developed their own innovative approaches.
Low-voltage electrolysis has been a favorite for its relative simplicity and low cost, but it has been the least successful in producing repeatable results and in proving a nuclear effect. Searches for excess heat are enticing and may get the attention of entrepreneurs because the heat may eventually lead to needed practical applications. However, heat is evanescent and makes a difficult proof for nuclear phenomena; in addition, heat teaches little about the nuclear mechanism.
On the other hand, searches for isotopic or elemental changes provide direct information about the mechanism and produce long-lasting nuclear evidence. Researchers who have conducted experiments in attempts to find isotopic or elemental changes have found that such experiments work more repeatably and more reproducibly.
The comprehensive Widom-Larsen theory of LENRs enables the following varied experimental methods and their results to be understood within a common conceptual framework. Among these methods listed below, an even more diverse collection of protocols and instrumentation choices exist for many of them.
|
| Basic Steps in LENR Experiments
Source: © New Energy Times. If you wish to reproduce any part of this index, please contact New Energy Times.
In 1991, Mahadeva Srinivasan made a list of the basic steps involved in most LENR experiments. Two decades later, the essential steps are the same and apply to most of the methods. Here is his list, slightly edited:
1. Choice of experimental method.
2. Choice of host metal/alloy: Pd, Ni, Ti, Zr, Mg, V, Nb-Ti, any hydrogen-storing alloy; even a high-temperature superconductor.
3. Preparation of samples: degassing, surface cleaning, annealing.
4. Choice of and loading of deuterium/hydrogen: electrolysis, gas, plasma, ion implantation, etc.
5. Measurement of degree of deuterium/hydrogen loading (or D(H)-to-metal atomic ratio): weighing, volume increase, resistivity, X-ray diffraction, etc.
6. Stimulation/triggering of reactions to create non-equilibrium conditions: current pulsing, thermal cycling, electrical discharge, application of electric or magnetic field, pressure changes, ultrasound, low-power laser, shock wave, projectile impact, etc. In many cases, particularly with electrolysis, anomalies do not occur when the system is in equilibrium.
7. On-line diagnostic analyses: heat, neutrons, X-rays, helium-4 (online), gamma rays, thermal imaging, acoustic or pressure waves, radio emissions.
8. Off-line diagnostics or post-experiment analysis: Isotopic anomalies, elemental changes, charged particles, neutrons via CR-39 with or without absorber, helium-4 (offline,) helium-3, tritium.
9. Theoretical interpretation, modeling, analysis.
Among these methods listed below, an even more diverse collection of protocols and instrumentation choices exist for each method.
|
| List of LENR Experimental Methods
Source: © New Energy Times. If you wish to reproduce any part of this index, please contact New Energy Times.
Category: Electrolytic Methods
Electrolysis in Heavy Water
Electrolysis in Light Water
Electrolysis with Low-Power Laser
Electrodiffusion with Double-Structure Cathode
High-Voltage Plasma Electrolysis in D2O or H2O
Electrolytic Co-Deposition
Thin-Film Electrolysis in Packed Bed
Thin-Film Electrolysis on Substrate
Category: Gas Methods
Gas Loading on Bulk Metal (Rod or Wire)
Gas Absorption Into Metal Nano-Powder
Gas Plasma - Glow Discharge
Gas Permeation Through Thin Films
Gas Permeation Through Thin Metals
Category: Unique Methods
Exploding Wires
Electron Beam Impact
Sonic Implantation
Biological Processes
Electromigration Through Solid-State Proton Conductors
Carbon Arc Experiments
Hydrogen Loading of Phenanthrene
|
| Category: Electrolytic Methods |
Electrolysis in Heavy Water
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
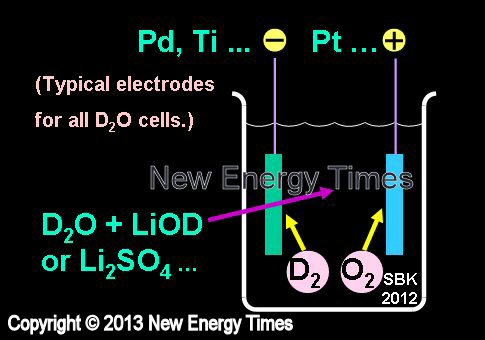
|
| Author Example |
Pons and Fleischmann |
| Typical Configuration |
Low-power electrolysis. Pd or Ti cathodes. Pt anodes. D2O with LiOD or Li2SO4. |
| Typical input power |
Less than 5 Watts |
| Typical duration |
Weeks to months |
| Typical Search |
Excess heat |
|
Electrolysis in Light Water
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
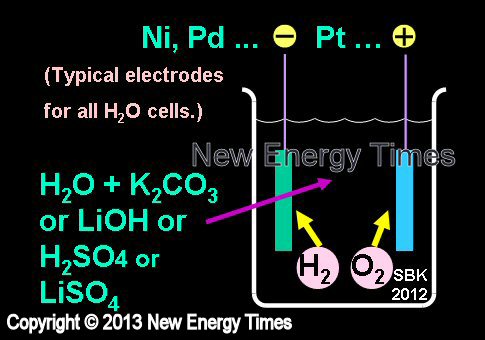
|
| Author Example |
Miley, Dash |
| Typical Configuration |
Low-power electrolysis. Ni cathodes. Pt anodes. H2O with K2CO3, LiOH or LiSO4 or H2SO4. |
| Typical input power |
Less than 5 Watts |
| Typical duration |
Days to weeks |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
Electrolysis with Low-Power Laser
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
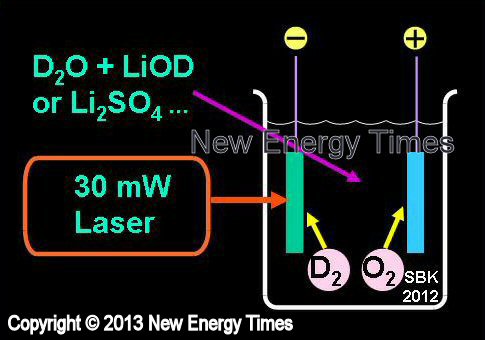
|
| Author Example |
Letts and Cravens, Violante |
| Typical Configuration |
Variation of low-power electrolytic experiment. Addition of ~30 mW laser beam amplifies excess-heat effect. |
| Typical input power |
Less than 5 Watts |
| Typical duration |
Days to weeks |
| Typical Search |
Excess heat |
|
Electrodiffusion with Double-Structure Cathode
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
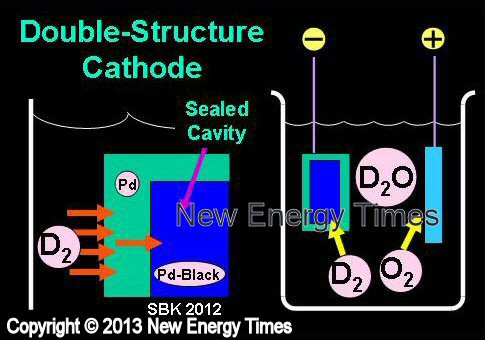
|
| Author Example |
Arata and Zhang |
| Typical Configuration |
Low-power electrolysis. Deuterium in D2O diffuses through the walls of a hollowed-out (double-structure) cathode filled with Pd-black powder. D2 gas permeates cathode walls. |
| Typical input power |
100 Watts |
| Typical duration |
Days to weeks |
| Typical Search |
Excess heat, 4He |
|
High-Voltage Plasma Electrolysis in D2O or H2O
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
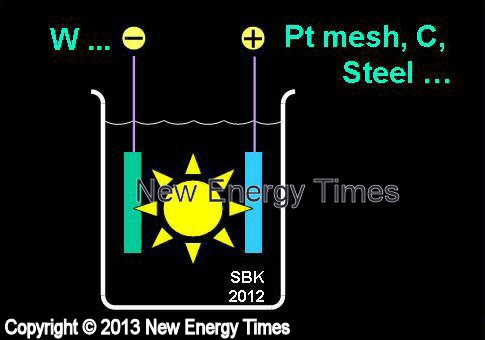
|
| Author Example |
Mizuno, Cirillo |
| Typical Configuration |
High-power electrolysis causes plasma formation. W cathodes. Anodes can be Pt mesh, carbon, steel. D2O with LiOD or H2O with any of LiOH, KOH, NaOH, K2CO3, Na2SO4, Na2CO3, H2SO4. |
| Typical input power |
100-800 Watts |
| Typical duration |
Minutes to hours |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
Electrolytic Co-Deposition
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
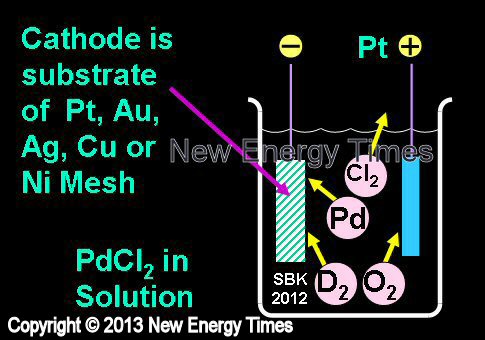
|
| Author Example |
Mosier-Boss, Miles |
| Typical Configuration |
Cathodes are substrates of Au, Ag or Pt. Anode is Pt. Solutions of PdCl2 and LiCl are added to D2O. Pd is deposited onto cathode in the presence of evolving D2. |
| Typical input power |
Less than 5 Watts |
| Typical duration |
Days |
| Typical Search |
Excess heat, energetic particles, neutrons |
|
Thin-Film Electrolysis in Packed Bed
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
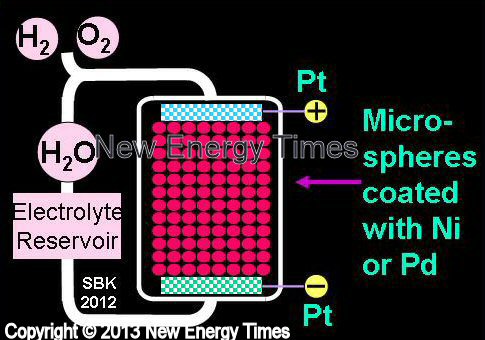
|
| Author Example |
Patterson and Miley |
| Typical Configuration |
Low-power electrolysis. A bed of microspheres coated with Ni or Pd, or both, are immersed in the electrolyte and compose the cathode. Pt electrodes. H2O with K2CO3, LiOH or LiSO4. |
| Typical input power |
Less than 5 Watts |
| Typical duration |
Hours to days |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
Thin-Film Electrolysis on Substrate
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
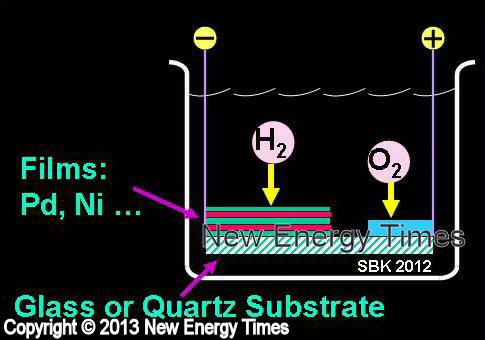
|
| Author Example |
Miley |
| Typical Configuration |
Glass/Quartz substrate. Cathode consists of multiple layers of Pd, Ni and other materials. Pt anode. |
| Typical input power |
Less than 5 Watts |
| Typical duration |
Hours to days |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
| Category: Gas Methods |
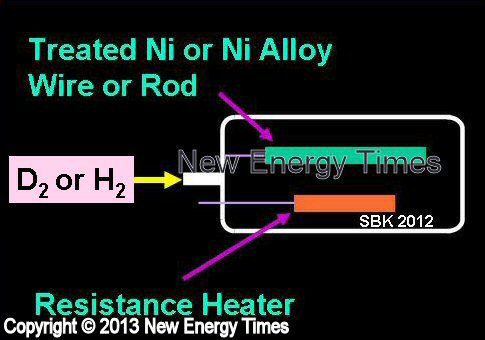
Gas Loading on Bulk Metal (Rod or Wire)
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Piantelli |
| Typical Configuration |
Pure nickel, nickel alloys or nickel-plated bars are placed in ultra-high-vacuum chamber, which is then filled with hydrogen gas. Initial heating is by resistance and gradually reduced as LENR heating increases. |
| Typical input power |
Tens of Watts |
| Typical duration |
Weeks to months |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
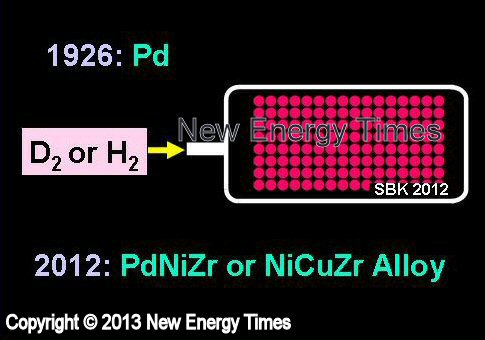
Gas Absorption Into Metal Nano-Powder
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Paneth and Peters, Arata |
| Typical Configuration |
Deuterium or hydrogen gas loads into a chamber filled with finely divided palladium or palladium nanopowder. |
| Typical input power |
Only pump power |
| Typical duration |
Days |
| Typical Search |
Search for transmutation, excess heat and helium-4 |
|
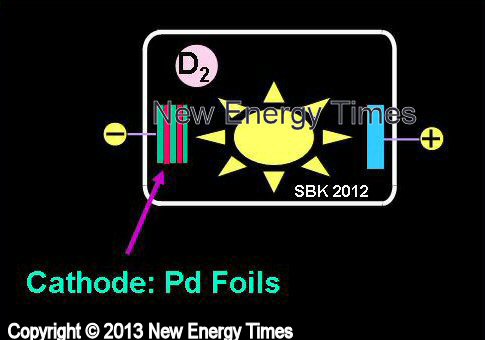
Gas Plasma - Glow Discharge
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Savvatimova |
| Typical Configuration |
Voltage applied to a chamber filled with deuterium or hydrogen gas, or a mixture, plus Ar, Xe, causes a plasma to form |
| Typical input power |
500-1,000 volts, 10-100 mA, (5-100 Watts) |
| Typical duration |
1-40 hours |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
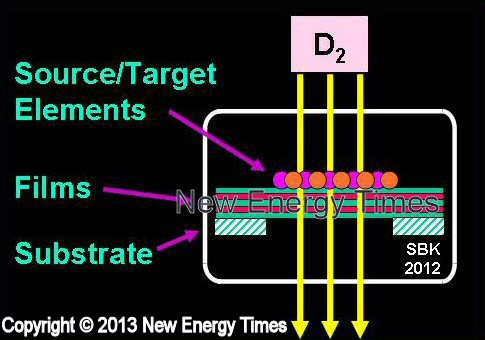
Gas Permeation Through Thin Films
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Iwamura, Higashiyama |
| Typical Configuration |
Deuterium gas drawn through multi-layer substrates containing palladium and target elements. |
| Typical input power |
Pump power and small electric heater, Pump: 400W Heater: 10 Watts |
| Typical duration |
10 days |
| Typical Search |
Search for nuclear emissions and transmutations |
|
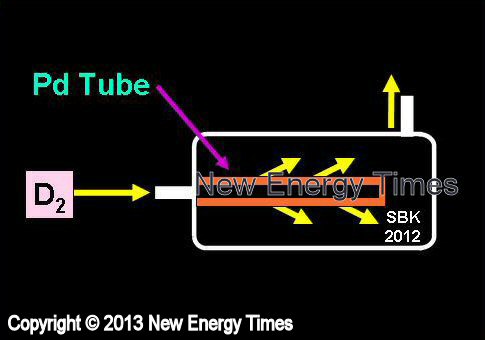
Gas Permeation Through Thin Metals
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Biberian, Fralick |
| Typical Configuration |
Deuterium or hydrogen gas is drawn through and loaded into or deloaded from thin sheets or tubes of palladium. |
| Typical input power |
Only pump power |
| Typical duration |
Days |
| Typical Search |
Excess heat, nuclear emissions |
|
| Category: Unique Methods |
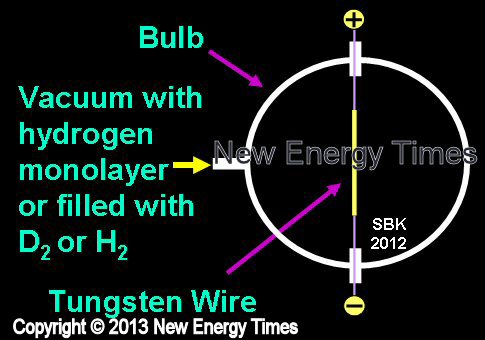
Exploding Wires
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Wendt and Irion, Kortkhonjia |
| Typical Configuration |
A high electric current is rapidly introduced to a thin wire. The wire disintegrates in a brilliant flash. |
| Typical input power |
Tens or hundreds of Watts |
| Typical duration |
Seconds to minutes |
| Typical Search |
Nuclear emissions and transmutations |
|
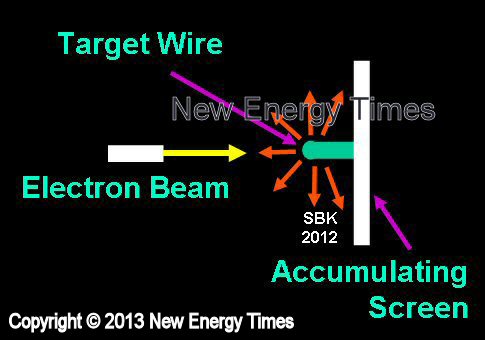
Electron Beam Impact
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Adamenko, Novikov |
| Typical Configuration |
Bombarding a target metal with high, short-pulse electron current |
| Typical input power |
Power of beam: 200J-2000J; Absorbed by a target: 20J-400J |
| Typical duration |
15ns-100 ns |
| Typical Search |
Nuclear emissions and transmutations |
|
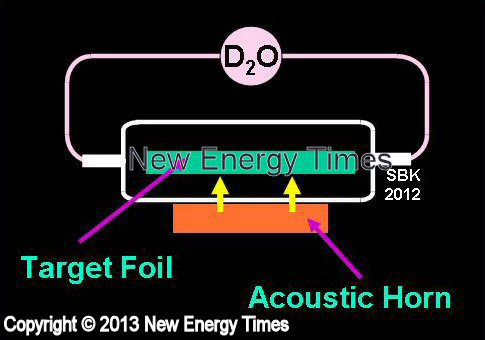
Sonic Implantation
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Stringham |
| Typical Configuration |
Acoustic waves are directed into an aqueous solution of D2O in the presence of a target metal. Bubbles form and collapse at the surface of the metal. |
| Typical input power |
16 Watts |
| Typical duration |
Hours |
| Typical Search |
Excess heat and helium-4 |
|
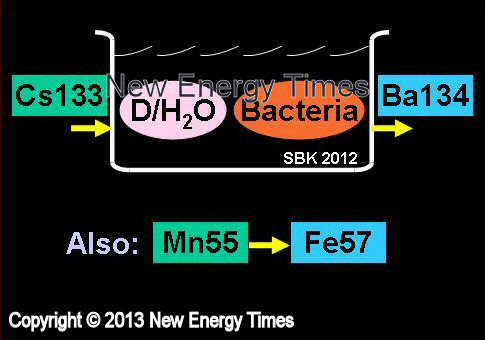
Biological Processes
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Vysotskii and Kornilova |
| Typical Configuration |
Dissolve MnSO4 in D2O or H2O containing bacteria. Rare iron isotope Fe57 produced and detected by gamma ray detection, Mossbauer method and TIMS mass-spectroscopy (synchronization of decrease of Mn55 concentration and increase of Fe57 concentration). |
| Typical input power |
None |
| Typical duration |
24-72 hours for the case of "online" ("clean") microbiological cultures like Escherichia coli (low efficiency of transmutation) and 50-100 days for microbe syntrophin association of thousands kinds of microbiological cultures (high efficiency of transmutation) |
| Typical Search |
Nuclear transmutations of stable (e.g. Mn55 to Fe57) and radioactive (e.g. Cs137 to Ba138) isotopes. |
|
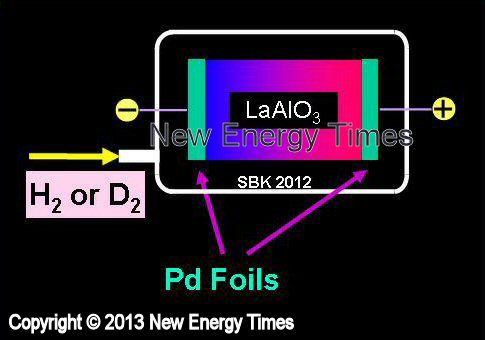
Electromigration Through Solid-State Proton Conductors
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Mizuno, Biberian |
| Typical Configuration |
Hydrogen or deuterium atoms caused to move across solid material when voltage is applied. |
| Typical input power |
100 mW-10 Watts |
| Typical duration |
Minutes to hours |
| Typical Search |
Excess heat |
|
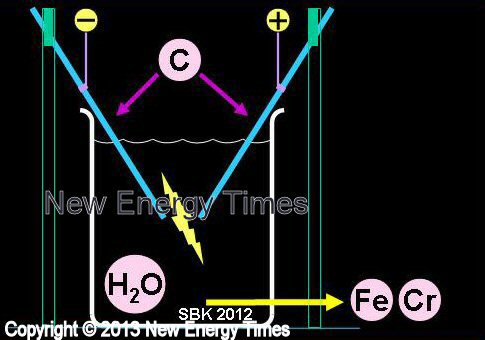
Carbon Arc Experiments
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Sundaresan and Bockris |
| Typical Configuration |
Carbon rods suspended in H2O. Transmutations found in detritus. |
| Typical input power |
10-30 amps |
| Typical duration |
Hours |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
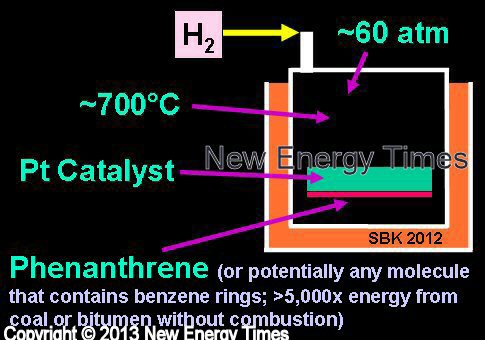
Hydrogen Loading of Phenanthrene
Source: © New Energy Times. If you wish to reproduce this image or table,
please contact New Energy Times.
|
| Author Example |
Mizuno |
| Typical Configuration |
Heavy oil is heated in high-pressure hydrogen gas with a metal catalyzer. |
| Typical input power |
500-1000 Watts |
| Typical duration |
Hours |
| Typical Search |
Excess heat, nuclear emissions and transmutations |
|
Change Log
May 26, 2013 Update
Exploding Wires: Image text updated: “Vacuum with hydrogen monolayer or filled with D2 or H2”
May 24, 2013 Update
Electrolysis in Heavy Water: Added “(Typical electrodes for all D2O cells.)”
Electrolysis in Heavy Water: Text in “Typical Configuration” corrected
Electrolysis in Light Water: Added “(Typical electrodes for all H2O cells.)”
Thin-Film Electrolysis in Packed Bed: "Pt anodes“ changed to “Pt electrodes”
Thin-Film Electrolysis on Substrate: Added: “Pt anode”
Gas Loading on Bulk Metal (Rod or Wire): Corrected to say “D2 or H2”
Petronuclear Hydrogen Loading of Phenanthrene: Change “benzine” to “benzene” ��
|
| |
|
|
|

